
Clean Cut Results Published in JAMA Surgery
Clean Cut results achieve a 34% relative risk reduction of surgical site infection
A new study published in JAMA Surgery demonstrates a 34% relative risk reduction of surgical site infection (SSI) after implementation of the Lifebox Clean Cut program.
The study ‘Scalability and Sustainability of a Surgical Infection Prevention Program in Low-Income Environments’ is based on data from 3,364 surgical patients across seven Ethiopian hospitals to assess SSI reduction after implementation of the Clean Cut program. Clean Cut reduces rates of SSI by strengthening adherence to six perioperative infection prevention standards.
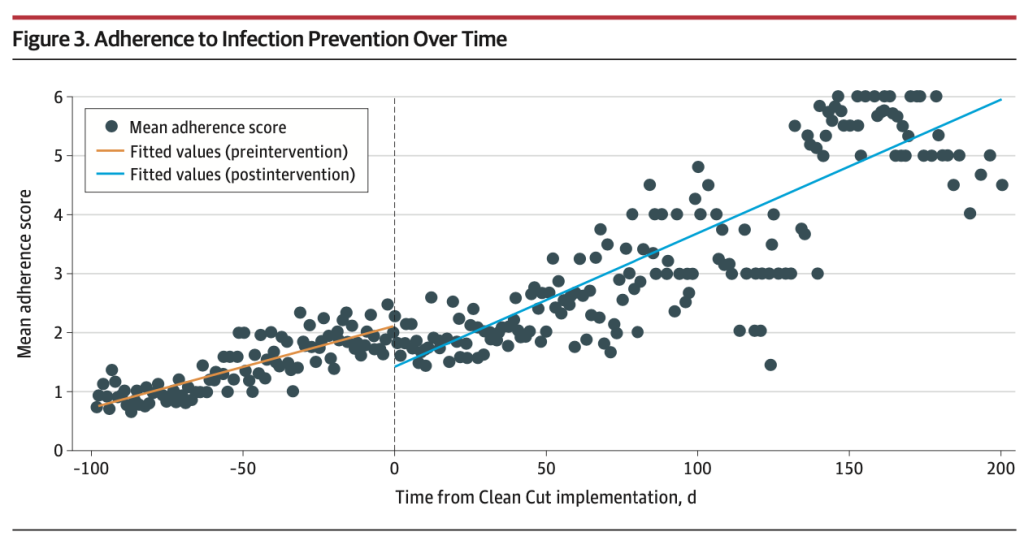
This study used a modified implementation strategy to support the scalability of Clean Cut. This included reducing external resource and programmatic input from Lifebox, structured education and training materials, and wider hospital engagement. This approach resulted in outcomes that matched our pilot study, with improved adherence to recognized infection prevention standards resulting in a reduction in SSIs.
There were signifiant improvements in adherence to the six Clean Cut perioperative infection prevention standards. Appropriate Surgical Safety Checklist use increased from 16.3% to 43.0%. Surgeon hand and patient skin antisepsis improved from 46.0% to 66.0%. Timely antibiotic administration improved from 17.8% to 39.0%. Surgical instrument sterility improved from 10.2% to 38.7%. Linen sterility improved from 12.8% to 35.5%. Gauze counting improved from 82.5% to 89.2%.
This builds on the evidence base of Clean Cut including results showing reduction in SSI and lasting impact published in the British Journal of Surgery and JAMA Surgery respectively. Clean Cut has now been implemented in partnership with 35 hospitals across seven countries.
©Yosef Amare Tsehayu/Lifebox, 2023. Operating room at Yekatit 12 Hospital Medical College, Ethiopia – a Clean Cut partner hospital
AUTHORS: Nichole Starr, MD, MPH, Natnael Gebeyehu, MD, Maia R. Nofal, MD, MPH, Jared A. Forrester, MD, Assefa Tesfaye, MD, MPH, Tihitena Negussie Mammo, MD, Thomas G. Weiser, MD, MPH, and Lifebox Clean Cut Collaborative.

